Regenerative Medicine practitioners worldwide have a rich history of performing prolotherapy. The injection of autologous stem cells for the treatment of chronic musculoskeletal pain can be viewed as the natural evolution of prolotherapy (5), and its proposed mechanism
of action, namely the regeneration of damaged or degenerated tissues through the triggering of the body’s own healing response, is perfectly aligned with the guiding principles of osteopathic medicine.
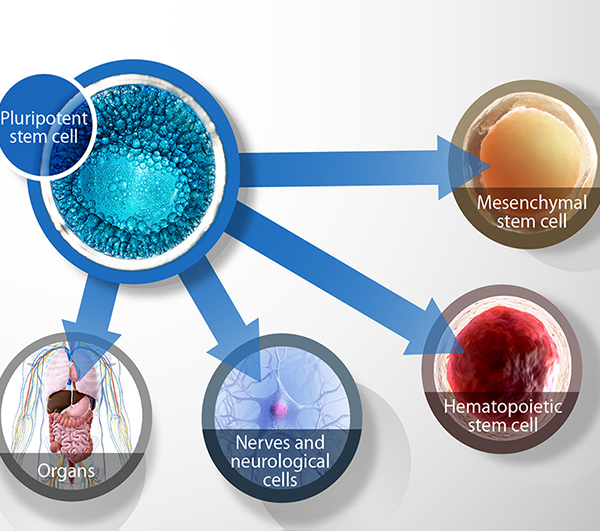
Stem cell science is fast gathering momentum in the field of regenerative medicine, and for good reason. Stem cells have the potential to regenerate into any type of body tissue. This swiftly changing landscape has seen many different stem cell types and technologies capture the public’s imagination including embryonic stem cells, tissue stem cells (mesenchymal stem cells), and more recently, induced pluripotent stem cells.
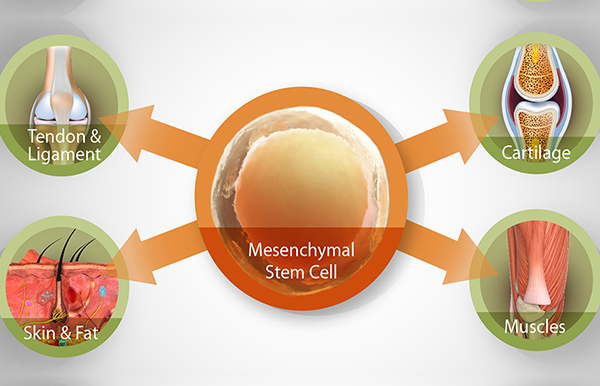
MSCs have been called “patient-specific drug stores for injured tissues” because of their ability to secrete bioactive factors and signals at variable concentrations in response to local microenvironmental cues. MSCs release a spectrum of anti-inflammatory, immunomodula-tory, and trophic factors that trigger the regeneration and healing of connective tissues through activation of stem cells endogenous to the site. Stem cell therapy is based on the premise that all musculo-skeletal structures contain populations of MSCs whose primary role is to maintain the health of their microenvironment; degeneration and pain occur when these populations either become depleted or lose their ability to function properly. Therefore, the goal of stem cell therapy is to repopulate degenerated structures and tissue beds with a robust population of viable MSCs. The site-specific injection of autologous stem cells has shown promise in musculoskeletal pain conditions such as osteoarthritis (6,7), sports/traumatic injury (8), low back and discogenic pain, neck pain with or without cervicogenic headaches (9), and osteonecrosis (10).
MSCs are found throughout the body in many tissue types, but they are particularly abundant and easily harvested from the medullary cavity of bones. MSCs can be easily concentrated from aspirated bone marrow using simple centrifugation, thereby rendering bone marrow aspirate concentrate (BMAC) suspended in platelet-rich plasma alone (11).
When MSCs are taken from bone marrow, and then injected directly into the problem area, we ‘trick’ the body into thinking that there has been a new injury without actually causing any tissue insult, and triggers a second chance at healing. In the case of advanced osteoarthritis where the population of stem cells has been depleted, we are repopulating the area with stem cells, and thereby restoring the body’s natural ability to heal itself.
But what are stem cells and what makes them so interesting?
Stem cells are found in the early embryo, the foetus, amniotic fluid, the placenta and umbilical cord blood. After birth and for the rest of life, stem cells continue to reside in many sites of the body, including skin, hair follicles, bone marrow and blood. In the growing body, stem cells are responsible for generating new tissues, and once growth is complete, stem cells are responsible for repair and regeneration of damaged and ageing tissues. The question that intrigues medical researchers is whether you can harness the regenerative potential of stem cells and be able to grow new cells for treatments to replace diseased or damaged tissue in the body.
Pluripotent Stem Cell
Why hasn't Stem Cell Therapy Work For Me?
When a patient questions or comes into our office seeking answers to why stem cell therapy did not work for their knee pain, we suggest that perhaps the answer was NOT:
- Because autologous blood stems cells were used when bone marrow stem cells should have been used or vice versa.
- or even Because the number of stem cells were inadequate.
It may be that the stem cell therapy has not yet had a chance to work because it has not been given the full chance to work. This may be a difficult idea for patients to understand, so here we will discuss why stem cell therapy may not have had the desired outcome yet for a patient with knee arthritis, and what we believe we can do to give it its best chance of working.
There are many reasons why we develop degenerative joint arthritis, physically stressful job, high level of activities, even including past histories of arthroscopic surgeries.
The main factor however is that the osteoarthritic joint is degenerating faster than the body can repair it. The body’s healing response is inflammation. Inflammation will turn on and continue staying on until the joint is healed. But what if the joint does not heal because the injury is too severe for the body to heal on its own? Then the inflammatory mechanism will be stuck in the “open” position, chronic inflammation will continue trying to heal something it can’t-referred to as 'runaway inflammation'.
A Chondrocyte is a cell that makes cartilage.
When you have many chondrocytes doing the same thing, they create a healing environment of positive, healing inflammation. This is what cartilage regeneration is, repair cells acting in a positive inflammation environment. But, when the chondrocytes can not complete the repair, it cannot shut off the inflammation, the inflammatory loop creates a toxic environment of oxidative stress. The stress factors create a diseased environment.
Stem cells do have the the ability to change this degenerative toxic environment into a regenerative healing environment. When stem cells are injected into a diseased joint they start talking to the repair cells, the blood cells, the inflammatory cells, and the native stem cells in a “reboot” command to restart the natural healing of the joint. This comes under the phenomena of “cell signalling".
However, the stem cells can be bogged down and the messages blurred by the oxidative stress caused by the chronic inflammation, so repair can take longer and be more challenging. It is this issue which becomes the greatest hurdle for any regenerative medicine practitioner.
While adult stem cells may be a promising cell source for cartilage regeneration, increasing evidence indicates that environmental preconditioning is a powerful approach in promoting stem cells’ ability to resist a harsh environment where the cells are in a constant oxidative state. So, even if the practitioner's intentions are 'pure' of heart, putting them into such an environment gives them little to no chance of survival.
The idea is to get the stem cells ready to more efficiently heal by changing their conditioning and the joint environment by removing oxidative stress in the joint.
An inflammatory milieu breaks down the cartilage matrix and induces chondrocyte apoptosis, resulting in cartilage destruction in patients with cartilage degenerative diseases, such as rheumatoid arthritis or osteoarthritis. Because of the limited regenerative ability of chondrocytes, defects in cartilage are irreversible and difficult to repair. Mesenchymal stem cells (MSCs) are expected to be a new tool for cartilage repair because they are present in the cartilage and are able to differentiate into multiple lineages of cells, including chondrocytes. Although clinical trials using MSCs for patients with cartilage defects have already begun, its efficacy and repair mechanisms remain unknown.

Figure 1
The inflammatory milieu is known to cause cartilage destruction by causing disturbances in the anabolic/catabolic balance of the cartilage matrix. The inflammatory milieu has also been understood to exert a suppressive action during the process of differentiation from progenitor MSCs to chondrocytes. Therefore, sufficient suppression of articular inflammation prior to a procedure may be important for efficient regeneration of the cartilage in regenerative therapies using MSCs. While continuous studies to develop more efficient techniques for chondrogenic differentiation using MSC-based cell therapies are important, it is necessary to establish a protocol for the improvement and maintenance of the intra-articular environment, where MSCs are transferred, to be suitable for cartilage regeneration.
In several clinical trials(12), MSCs injected into the joints by the least-invasive intra-articular method, and were expected to differentiate into chondrocytes locally in the joint. Whether or not the intra-articular environment of the joints was suitable for chondrogenic differentiation of MSCs is a major concern. As mentioned , cartilage regenerative therapy is likely to be indicated when the affected joints in patients with OA and RA present with inflammatory reactions. However, the inflammatory milieu, including proinflammatory cytokines, strongly inhibits MSC differentiation into chondrocytes. On the other hand, it has been demonstrated that the influence of inflammation (pro inflammatory cytokines) on MSC is differentiation into osteoblasts, resulting in the formation of calcifications. These findings may indicate the risk of developing osteophytes and ectopic calcifications when MSCs are injected into the joint in an inflammatory milieu. Therefore, sufficient suppression of articular inflammation prior to any procedure is important for efficient cartilage regeneration while using MSCs in cartilage regenerative therapy. This can be achieved by a combination of an existing anti-inflammatory agent or a biologic targeting proinflammatory cytokine and a chondro-enhancing agent (Figure 1).
A joint does not try to repair one part of itself, it tries to repair its whole self
In Japan, researchers looked at what was the impact of menisectomy, the surgical removal of meniscal tissue from the knee and why this meniscal removal caused the whole knee to begin an accelerated degenerative process.
What they found in this study was the body’s rapid response to repair the meniscus, not only tries to regenerate meniscal tissue damaged by the surgery, but also tried to prevent damage to the articular cartilage of the knee by sending repair cells there. Why? Because without the meniscus the cartilage covering the articular surface is subject to accelerated load forces.
The researchers concluded that the remaining meniscus and the articular cartilage of the knee tried, in vain, to work together to regenerate the meniscus and protect the cartilage.3
In other words, the knee tried to reinforce the articular cartilage because it knew the meniscus was gone.
Consider other structures of the knee? They are also part of the whole knee environment?
We have previously discussed the research practitioners explored in the use of simple Dextrose Prolotherapy for problems of knee osteoarthritis, while the documented success of treating knee osteoarthritis is a great validation for the treatment, the best part of the research was the acknowledgement that these practitioners would have had greater success had they treated the whole knee.
Platelet Rich Plasma contains a myriad of substances that stimulate healing:
- Platelet-Derived Growth Factor (PDGF) Attracts immune system cells to the area and stimulates them to proliferate. Has been shown to enhance ligament and tendon healing.
- Transforming Growth Factor-8 (TGF-8) Secreted by and affects all major cell types involved in healing. Similar affects as PDGF.
- Vascular Endothelial Growth Factor (VEGF) Helps new blood vessel formation, thereby increasing vascularity in injured areas.
- Fibroblast Growth Factor (FGF) promotes the growth of the cells involved in collagen and cartilage formation.
- Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine..
Stromal cell-derived factor-1α (SDF-1) is a chemokine (signalling protein secreted by cells) that plays a major role in the homing of CD34(+) mesenchymal stem cells. Studies employing SDF-1 have demonstrated its therapeutic potential in tissue engineering. During injury, cells from the injured organ highly express SDF-1, this leads to recruitment and retention of circulating CD34(+) progenitor cells at the injury site.
Stem cells are at the core of the tissue regeneration process. They are required to rebuild and repair damaged tissue at an injury site. Platelets release several growth factors, such as SDF-1α, that function to induce migration of stem cells to the damaged tissue during the healing process. PRP provides these growth factors in high concentrations. In addition, our PRP preparations also contain peripheral blood mesenchymal stem cells isolated directly from the blood draw during processing (<1%), which can directly support tissue regeneration. Numerous studies have shown that PRP enhances the effects of Stem Cell Therapy and autologous PRP can be more effective in promoting the chondrogenesis of BMSCs.1,2
2. Kasten P, Vogel J, Beyen I, Weiss S, Niemeyer P, Leo A, Lüginbuhl R. Effect of platelet-rich plasma on the in vitro proliferation and osteogenic differentiation of human mesenchymal stem cells on distinct calcium phosphate scaffolds: the specific surface area makes a difference. J Biomater Appl. 2008 Sep;23(2):169-88. Epub 2008 Jul 16.
3. Hiyama K, Muneta T, Koga H, Sekiya I, Tsuji K. Meniscal regeneration after resection of the anterior half of the medial meniscus in mice. J Orthop Res. 2016 Nov 2. doi: 10.1002/jor.23470.
4. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with moderate knee osteoarthritis: a single-arm study with 6 months follow up. Therapeutic Advances in Musculoskeletal Disease. 2015;7(2):35-44.
5. DeChellis DM, Cortazzo MH. Regenerative medicine in the eld of pain medicine: Prolotherapy, platelet-rich plasma therapy, and stem cell therapy—Theory and evidence. Techniques in Regional Anesthesia and Pain Management. 2011;15(2):74–80.
6. Goldberg, VM. Stem cells in osteoarthritis. HSS J. 2012;8(1):59-61.
7. Luyten FP. Mesenchymal stem cells in osteoarthritis. Curr Opin Rheumatol. 2004;16(5):599-603.
8. Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009;28(1):1-11.
9. Adelson H. Bone Marrow and Adipose Derived Autologous Stem Cells for the Treatment of Chronic Musculoskeletal Pain. Presented at: 25th Annual Meeting of the American Academy of Pain Management; September 2014; Phoenix, AZ.
10. Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5:296.
11. Adelson H. Autologous stem cell therapy: a naturopathic approach to the treatment of chronic musculoskeletal pain conditions, part II of II. Pain Practitioner. 2015;25(4):40-43.
12. Masahiro Kondo, Kunihiro Yamaoka and Yoshiya Tanaka: Acquiring Chondrocyte Phenotype from Human Mesenchymal Stem Cells under Inflammatory Conditions. Int J Mol Sci. 2014 Nov; 15(11): 21270–21285.